HKGP greatly values the privacy and the security of every participant’s data. The collection procedure for all personal and genomic data complies with the Hong Kong law, Personal Data (Privacy) Ordinance, Cap. 486, and all data will be kept strictly confidential.
1.Data Storage
HKGI will classify your personal information, medical records, and genomic data as confidential information, and properly store them in HKGI’s database located in Hong Kong.
2.Data Access
Only authorised designated Project team staff can access your medical records and genomic data for analysing your conditions and preparing reports. The personal data that can identify you will only be used for your clinical diagnosis and care.
You can also make a request to HKGI to access your genomic information.
HKGI will share only your de-identified data (data that does not include personal information) for research purpose. Qualified research organisations and researchers must seek approval from HKGI’s Institutional Review Board to view and analyse de-identified data on HKGI’s designated platform.
What is Institutional Review Board (also known as Research Ethics Committee)?
When a researcher or research institution applies to HKGI for the use of relevant data for research, the Institutional Review Board will examine and approve the study based on relevant guidelines, mainly predicated on ensuring the benefits to patients or to Hong Kong’s future medical development. Eligible research institutions and personnel (local and non-local) may include:
- Non-profit bodies (such as universities, research organisations); and
- Commercial enterprises (such as pharmaceutical manufacturers).
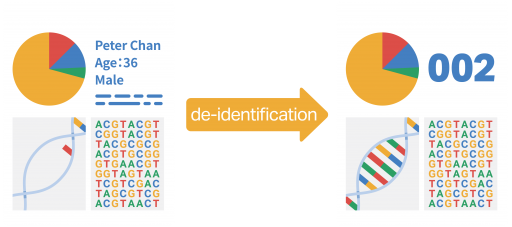
What is “de-identified data”?
“De-identified data” is data that does not contain any of your personal information (such as name, date of birth, identification document number, hospital number, and other personal details), which will have been removed by HKGI and replaced with an identification code.